|
 |
Gliadel Wafers
 GLIADEL® Wafer (polifeprosan 20 with
carmustine implant) is indicated in patients with newly
diagnosed high-grade malignant glioma as an adjunct to surgery
and radiation. It is also indicated in patients with recurrent
glioblastoma multiforme as an adjunct to surgery. It should not
be given to patients who have demonstrated a previous
hypersensitivity to carmustine or any of the components of
GLIADEL Wafer. Patients undergoing craniotomy for malignant
glioma and implantation of GLIADEL Wafer should be monitored
closely for known complications of craniotomy, including
seizures, intracranial infections, abnormal wound healing, and
brain edema. Cases of intracerebral mass effect unresponsive to
corticosteroids have been described in patients treated with
GLIADEL Wafer, including cases leading to brain herniation.
Carmustine, the active component of GLIADEL Wafer, can cause
fetal harm when administered to a pregnant woman. It is
recommended that patients receiving GLIADEL Wafer discontinue
nursing.
GLIADEL® Wafer (polifeprosan 20 with
carmustine implant) is indicated in patients with newly
diagnosed high-grade malignant glioma as an adjunct to surgery
and radiation. It is also indicated in patients with recurrent
glioblastoma multiforme as an adjunct to surgery. It should not
be given to patients who have demonstrated a previous
hypersensitivity to carmustine or any of the components of
GLIADEL Wafer. Patients undergoing craniotomy for malignant
glioma and implantation of GLIADEL Wafer should be monitored
closely for known complications of craniotomy, including
seizures, intracranial infections, abnormal wound healing, and
brain edema. Cases of intracerebral mass effect unresponsive to
corticosteroids have been described in patients treated with
GLIADEL Wafer, including cases leading to brain herniation.
Carmustine, the active component of GLIADEL Wafer, can cause
fetal harm when administered to a pregnant woman. It is
recommended that patients receiving GLIADEL Wafer discontinue
nursing.
Communication between the surgical resection cavity and the
ventricular system should be avoided to prevent the wafers from
migrating into the ventricular system and causing obstructive
hydrocephalus. If a communication larger than the diameter of a
wafer exists, it should be closed prior to wafer implantation.
CT and MRI of the head may demonstrate enhancement in the brain
tissue surrounding the resection cavity after implantation of
GLIADEL Wafer. This enhancement may represent edema and
inflammation caused by GLIADEL Wafer or tumor progression.
The short-term and long-term toxicity profiles of GLIADEL Wafer
when given in conjunction with chemotherapy have not been fully
explored.
 The following 4 categories of adverse
events are possibly related to treatment with GLIADEL Wafer:
The following 4 categories of adverse
events are possibly related to treatment with GLIADEL Wafer:
Seizures: In the initial surgery trial, the incidence of
seizures was 33.3% in patients receiving GLIADEL Wafer and 37.5%
in patients receiving placebo. Grand mal seizures occurred in 5%
of GLIADEL Wafer–treated patients and 4.2% of placebo-treated
patients. The incidence of seizures within the first 5 days
after wafer implantation was 2.5% in the GLIADEL Wafer group and
4.2% in the placebo group.
In the surgery for recurrent disease trial, the incidence of
post-operative seizures was 19% in both patients receiving
GLIADEL Wafer and placebo. In this study, 12/22 (54%) of
patients treated with GLIADEL Wafer and 2/22 (9%) of placebo
patients experienced the first new or worsened seizure within
the first 5 post-operative days.
The median time to onset of the first new or worsened
post-operative seizure was 3.5 days in patients treated with
GLIADEL Wafer and 61 days in placebo patients.
Brain Edema: In the initial surgery trial, brain edema was noted
in 22.5% of patients treated with GLIADEL Wafer and in 19.2% of
patients treated with placebo. Development of brain edema with
mass effect (due to tumor recurrences, intracranial infection,
or necrosis) may necessitate re-operation and, in some cases,
removal of GLIADEL Wafer or its remnants.
Healing Abnormalities: The following healing abnormalities have
been reported in GLIADEL Wafer clinical trials: wound
dehiscence, delayed wound healing, subdural, subgaleal or wound
effusions, and cerebrospinal fluid leak. In the initial surgery
trial, healing abnormalities occurred in 15.8% of GLIADEL
Wafer–treated patients and in 11.7% of placebo recipients.
Cerebrospinal fluid leaks occurred in 5% of GLIADEL Wafer
recipients and 0.8% of those given placebo.
During surgery, a water-tight dural closure should be obtained
to minimize the risk of cerebrospinal fluid leak. In the surgery
for recurrent disease trial, the incidence of healing
abnormalities was 14% in GLIADEL Wafer–treated patients and 5%
in patients receiving placebo wafers.
Intracranial Infection: In the initial surgery trial, the
incidence of brain abscess or meningitis was 5% in patients
treated with GLIADEL Wafer and 6% in patients receiving placebo.
In the recurrent setting, the incidence of brain abscess or
meningitis was 4% in GLIADEL Wafer patients and 1% in patients
receiving placebo.
| |
|
|
| |
 |
|
| |
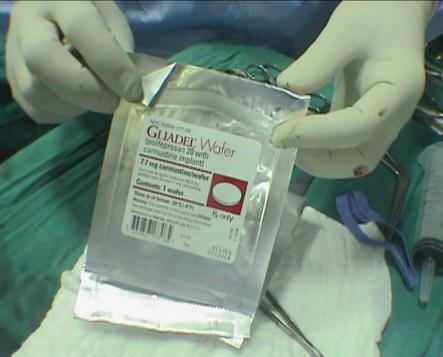
Gliadel
wafers.
|
|
|
 |
 |
|
This site is non-profit directed
to medical and neurosurgical audience to share
problems and solutions for brain tumors
diagnosis and treatment modalities.
Author of the
site.

Prof. Munir A. Elias MD., PhD.
Facts of life

When entering the soul of the human, there is a
great discrepancy about the value of timing of
the life. Some are careless even about the
entire of their existence and others are
struggling for their seconds of life.
Quality of life

It plays a major impact in decision making from
the patient. Here come the moral, ethics,
religious believes and the internal motives of
the patient to play a major hidden role in his
own survival.
|
|
|


